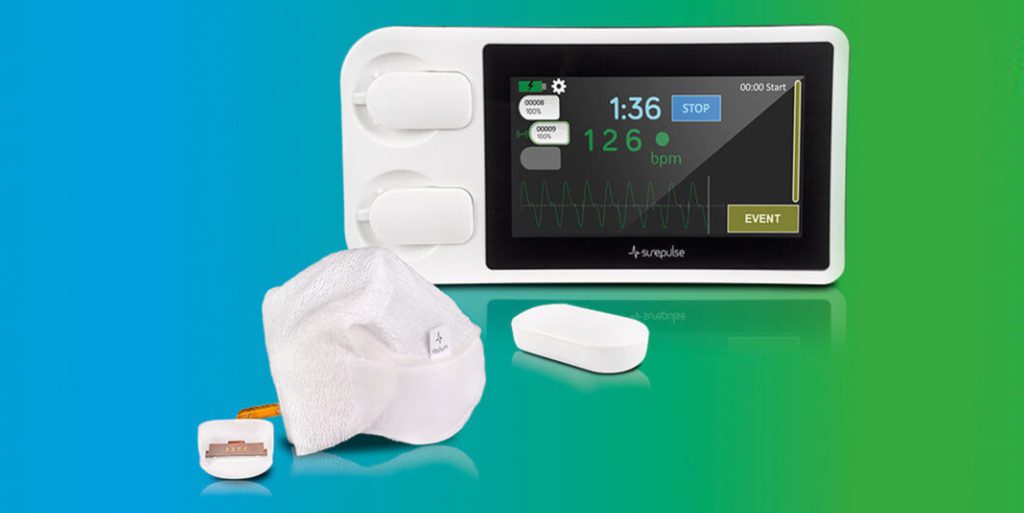
SurePulse Medical Ltd, a medical device company based in Nottingham, U.K., has announced today that it has received US FDA 510(k) clearance for its SurePulse VS Newborn Heart Rate Monitor.
The wireless SurePulse VS is specifically designed to be used straight after birth in vulnerable newborn babies. The aim of the heart rate monitor is to give clinicians the right information at the right time to guide optimal neonatal care, something that existing technologies struggle with in this population. The device offers new possibilities in patient care. In addition to enhancing the life chances for newborn babies (and optimising newborn care), the wireless capabilities allows for placement flexibility and easier parent bonding with the newborn baby. It has a touchscreen interface which facilitates event recording for audit, training and governance. The single-use cap, which integrates an optical sensor, is thermally protective and provides attachments for breathing apparatus.
James Carpenter, CEO of SurePulse, said “Our vision is to revolutionize neonatal vital sign monitoring and give clinicians the tools to provide optimal newborn care. We are thrilled to have achieved this important milestone on this journey as the US is a key market for growth, with nearly 400,000 premature births a year. Historically, neonatal care has been significantly underserved with little investment in new technologies, and so this is a real win for clinicians, babies and their parents. SurePulse VS has been designed from the ground up with the baby firmly in mind.”
About SurePulse
SurePulse Medical Ltd is a joint venture between the University of Nottingham (UoN) and Tioga Ltd, a spin-out of the UoN Faculties of Engineering and Medicine. The company has developed and patented technology for monitoring the vital signs of newborn babies. The company has won multiple awards (Medilink East Midlands Startup of the Year 2018 and Innovation Award 2019) recognising the achievements of its 16-strong team of engineering, clinical, commercial and operational excellence.